US researchers have developed a particular membrane inspired by biological processes capable of eliminating the main obstacle to the commercialization of lithium-sulfur ion batteries, a technology that has the potential to quintuple the range of electric cars.
In a scientific article published in Nature Communications, researchers from the Michigan Center for Materials Characterization of the University of Michigan have demonstrated a technology that allows to overcome one of the main obstacles to the development of lithium-sulfur ion batteries suitable for use in electric vehicles.
Lithium-Sulfur batteries, as the name suggests, use sulfur as the material for the cathode, one of the most abundant and inexpensive elements on Earth. Not only that: sulfur offers up to 10 times the specific capacity of the normal cathodes used in lithium-ion batteries, with the theoretical possibility of making batteries with an energy density of 2500 Wh / kg, a potential that would allow to reach batteries. much lighter, less expensive and with autonomy up to 5 times higher than the best technology available today. However, there is a big limitation: current Lithium-Sulfur batteries have an extremely limited duration, of the order of about ten charging cycles.
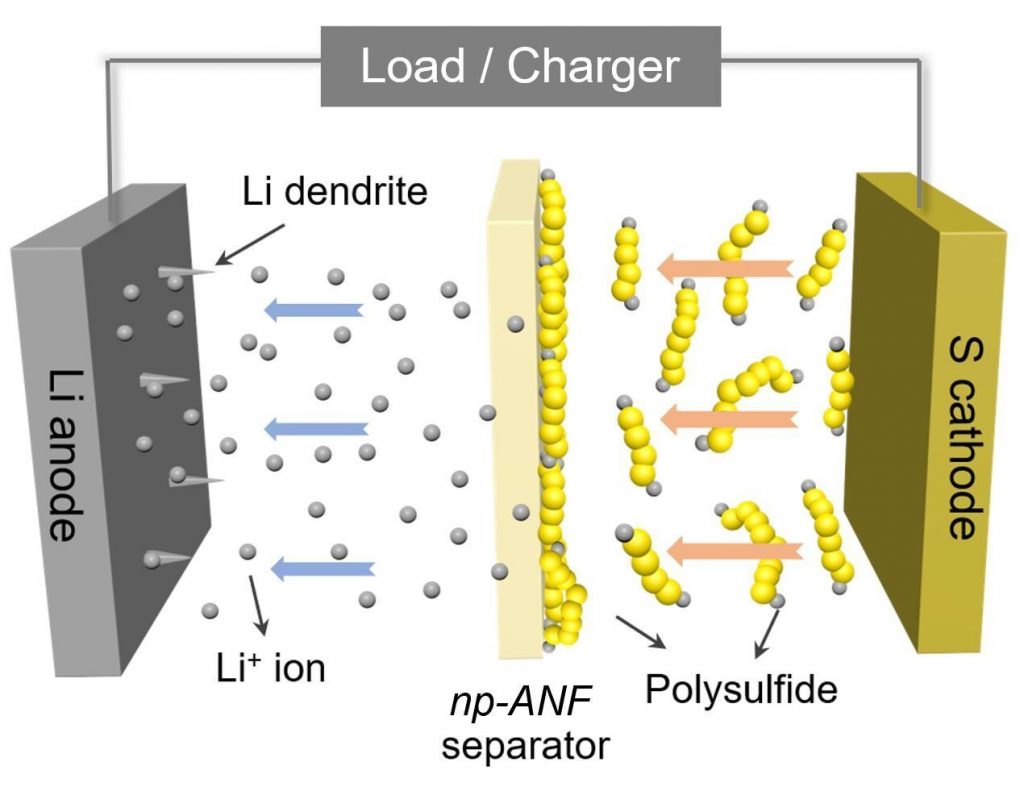
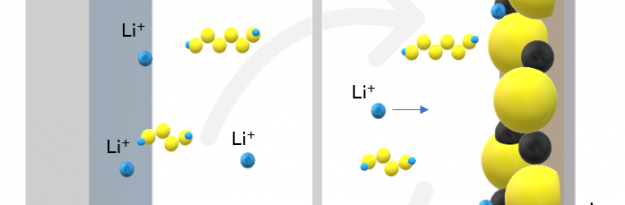
The research conducted by Nicholas A. Kotov’s team has developed a particular porous membrane capable of blocking the main cause behind the reduced longevity of lithium-sulfur batteries, i.e. the formation of lithium polysulphide molecules in the cathode, which migrate towards the anode, covering it, and ending up electrically isolating it, thus blocking the operation of the cell. The researchers drew inspiration from porous cell membranes to create a barrier capable of letting lithium ions pass but blocking polysulfides.
To do this, they developed a membrane made of aramid nanofibers, the same synthetic material used for the production of kevlar. The pores of the membrane capture the negatively charged polysulfides which in turn are able to repel the polysulfides that continue to form on the cathode, letting the positively charged lithium ions pass instead. Furthermore, the stiffness of the nanofibers prevents the formation of dentrites on the anode, another factor that traditionally limits Lithium-Sulfur batteries.
The technology, according to the researchers, solves the main limitations of lithium-sulfur batteries without compromising other parameters, such as charge density. The material developed is suitable for the temperatures reached by the cells for the automotive sector and would be able to offer up to 1000 charging cycles even with fast charging, sufficient to guarantee a battery longevity of the order of 10 years. The innovation featured in the study was patented by the University of Michigan, and Kotov is founding a start-up to bring the technology to the battery market.